We Are Passionate About Saving Lives With Pioneering Proven Diagnostics

Proven Expertise
Founded in 2002, Cellex Incorporated is a biotech company that develops technologies, instruments and assays for testing of human diseases and conditions, particularly for point-of-care (POC) professional healthcare settings.
20+
Years of experience in IVD product development
18
U.S. Government grants, including 13 from the National Institutes of Health
50+
Diagnostic products for various applications

Our Industry-Leading Technologies
Lateral Flow Chromatographic Immunoassay
Lateral Flow Chromatographic Immunoassay is a rapid and user-friendly diagnostic test that detects the presence of a specific target substance in a liquid sample. Cellex q Rapid Test and Lateral Flow Rapid Test utilize this technology for quick and accurate diagnosis of various diseases.
Real-Time PCR
(qPCR)
Real-Time PCR, also known as qPCR, is a powerful technique used to measure the amount of a specific DNA segment in a sample in real time. Our qPCR diagnostic instruments and reagents can be used to detect various diseases.
Homogeneous Biochemiluminescence Assay
HBA involves the measurement of light emission resulting from biochemical reactions, providing sensitive and accurate testing results. With the use of HBA Instruments, HBA q Rapid Test can be used to detect various diseases, such as influenza Types A and/or B virus, etc.
Explore Our Innovative Diagnostic Solutions
We have a lineage of more than 50 pioneering IVD diagnostic products, offereing precise diagnostics at the point-of-care (POC) for a wide range of applications. Our cutting-edge technologies offer healthcare professionals reliable and accurate tools.

Lateral Flow Diagnostic Products

HBA Instruments and Reagents

qPCR Instruments and Reagents
Latest News & Articles
-
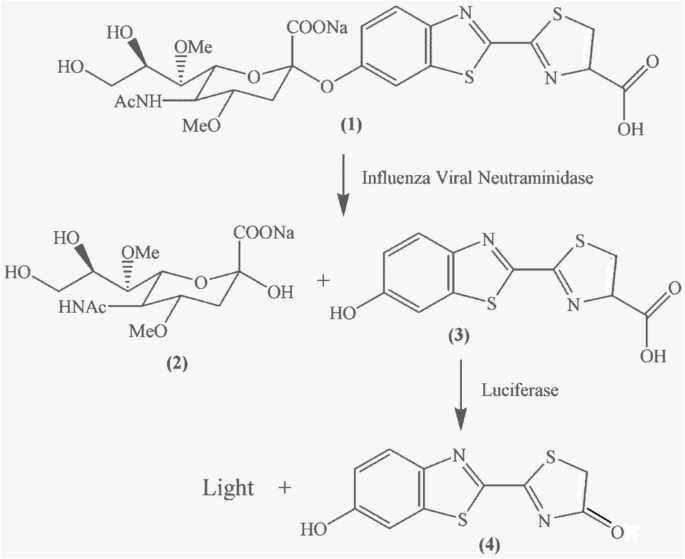
A rapid influenza diagnostic test based on detection of viral neuraminidase activity
Current methods used for diagnosis of acute infection of pathogens rely on detection of nucleic acids, antigens, or certain classes of antibodies such as IgM. Here we report a virus enzyme assay as an alternative to these methods for detection of acute viral infection.
-
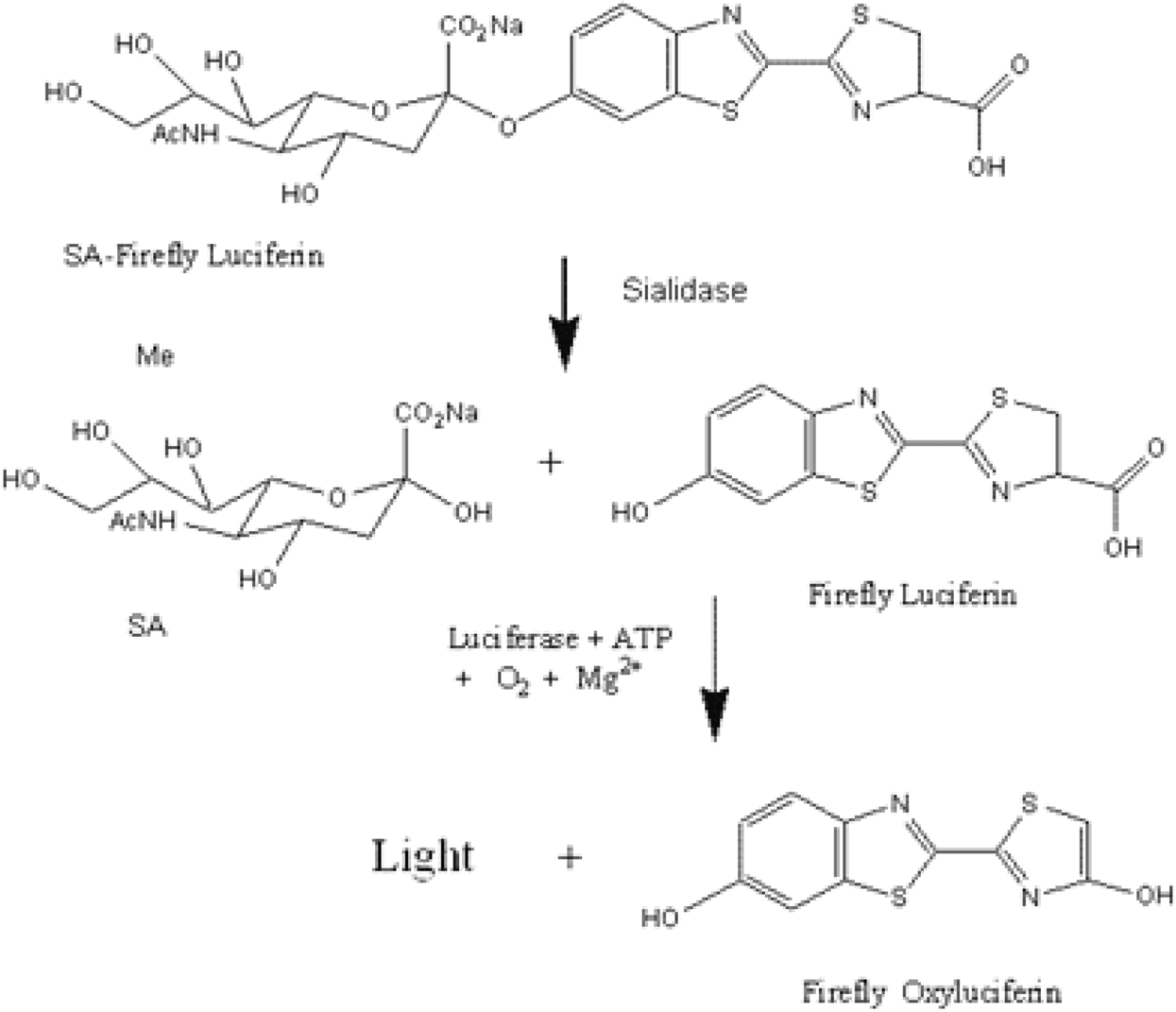
A Biochemiluminescent Sialidase Assay for Diagnosis of Bacterial Vaginosis
Bacterial sialidase appears to play an important role in bacterial biofilms on vaginal epithelium, a condition closely associated with BV. Here, we report a biochemiluminescent sialidase assay that uses a substrate derivatized with firefly luciferin.
-
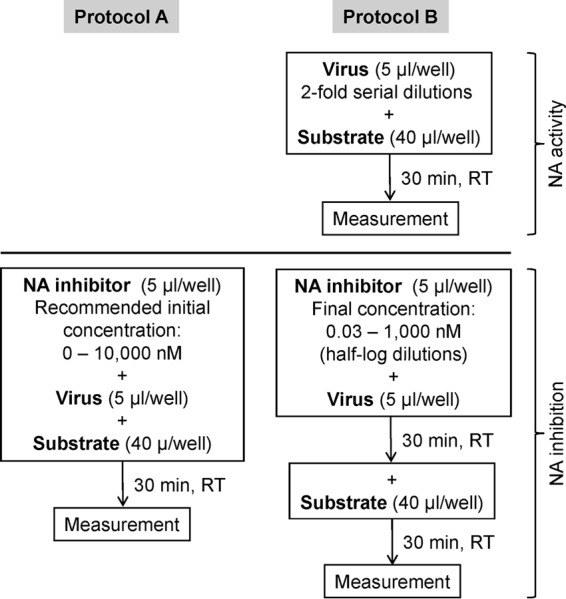
Bioluminescence-Based Neuraminidase Inhibition Assay for Monitoring Influenza Virus Drug Susceptibility in Clinical Specimens
The QFlu prototype bioluminescence-based neuraminidase (NA) inhibition (NI) assay kit was designed to detect NA inhibitor (NAI)-resistant influenza viruses at point of care. Here, we evaluated its suitability for drug susceptibility assessment at a surveillance laboratory.

Our Vision
Be an Innovative, Globally Competitive and Well-Respected Diagnostic and Biopharmaceutical Company.
Our Mission
Create Value and Opportunities for Our Employees, Shareholders, and Partners by Bringing Innovative Solutions to Our Customers.







